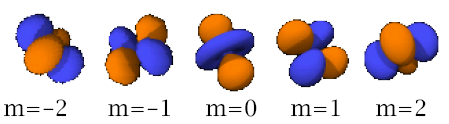Thursday, August 30, 2007.
I tried to do 1st part then realised that it will take forever.So this is just 2 pages of it.
Crystal Field Theory or CFT in short, is a simple but elegant theory to describe the behaviour of coordination complexes and how it changes based on the ligands and the orbitals.
Before one starts on understanding CFT, one starts on understanding the concept of ligand bonding. A ligand (e.g. H2O or NH3) forms a dative (coordinate covalent) bond with the usually empty d-orbital of the transition metal, and thus binds to it. Thus there is a subtle but important difference between CuSO4 (aq) and CuSO4. 5H2O.
But that isn’t enough. As we all learnt, the empirical formula of hydrated copper (II) sulphate is “CuSO4. 5H2O”, which is deemed correct by many people. But to be more precise, what CuSO4. 5H2O implies is that in the lattice of the CuSO4 molecule, there are on average 5 H2O molecules “trapped” in the lattice structure, which is not true, as experimentally determined. But through experiments the empirical formula of hydrated copper (II) sulphate is not CuSO4. 5H2O but rather [Cu (H2O)4]SO4. 5H2O. Note that in this formula, it clearly shows that the Cu2+ ion is bonded to 4 H2O molecules, and in the whole crystal one H2O molecule is trapped per every [Cu (H2O)4]2+ and SO42- ion. That is why although there is a compound called CrCl3. 6H2O, there are actually 3 different salts of it, known as isomers, with different colours in solution and even chemical properties.
For a compound/ion to be a ligand it must most obviously have a lone pair (or else how would it ever form a coordinate covalent bond?). They are Lewis bases (remember? Lewis bases are electron-pair donors), and the most common ligand is H2O and NH3. There are many more of them, like CN- and S2O32-, and I will be elaborating later about some properties of them (in the 2nd part).
Now back to the transition metal ion. I believe you know how an s-orbital looks like? A p-orbital? To refresh your minds:
(Pictures generously taken from http://en.wikipedia.org/wiki/Atomic_orbital (since it is free to take, as stated so in the license agreement))
Let’s focus on the d-orbitals. Notice the shapes of the orbitals. All of them actually look nearly the same except 2. (Pictures from www.chemsoc.org/VISELEMENTS/orbital/orbital_d.html) In order- from dxy to dxz to dyz to dx2-y2 to dz2:
The 1st thing that is obvious is that the 3dz2 orbital looks different from the other 4 orbitals-it has only 2 lobes, both on the z-axis, and an inner region of high electron density. Next, one can also realize that the 3dxy, 3dxz and 3dyz orbitals look exactly the same except for the axes (all have lobes of high electron density in between the axes), while the 3dx2-y2 orbital has its 4 lobes (regions of high electron density) all on the x and y axes. This will come in important later on.
Now lets try to figure out the significance in the shape of the orbitals with respect to the energy level. These are the orbitals binding to the electron pairs of the ligands, and as known, the lower the energy level the more stable the bond formed. Since in an isolated ion (aka no ligands) the d atomic orbitals are degenerate (have the same energy levels), thus one will expect it to be degenerate also when bonded to ligands. Not true. When the electron pair in the ligands bond, for the dx2-y2 and the dz2 orbitals the electron is forced to go near the region of higher electron density (since the electron pair of the ligands bond at the axes), thus it will experience more electrostatic repulsion, and thus the energy of the electron increases due to like charges.
Labels: chem
{ 9:55 PM }
narcissism.
lumpy.
4B '08, RISE
RJCE, Alchemy
materialist.
oh am I? *scratches head*
music.
shostakovich. mahler. brahms. rachmaninoff. vaughan williams. bruckner. bach. tchaikovsky.
はなせ.
でぐち.
4B '08!
RISE!
Others
memories.
August 2007 September 2007 October 2007 November 2007 December 2007 January 2008 February 2008 March 2008 April 2008 May 2008 June 2008 July 2008 August 2008 September 2008 October 2008 November 2008 December 2008 January 2009 February 2009 March 2009 April 2009 May 2009 June 2009 July 2009 August 2009 September 2009 October 2009 November 2009 December 2009 January 2010 March 2010
thanks.
Layout by BAKEDPOTATOE, with help from sm3no for the image and fonts, Print Dashed and Violation.