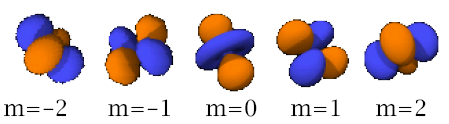Thursday, August 30, 2007.
I tried to do 1st part then realised that it will take forever.So this is just 2 pages of it.
Crystal Field Theory or CFT in short, is a simple but elegant theory to describe the behaviour of coordination complexes and how it changes based on the ligands and the orbitals.
Before one starts on understanding CFT, one starts on understanding the concept of ligand bonding. A ligand (e.g. H2O or NH3) forms a dative (coordinate covalent) bond with the usually empty d-orbital of the transition metal, and thus binds to it. Thus there is a subtle but important difference between CuSO4 (aq) and CuSO4. 5H2O.
But that isn’t enough. As we all learnt, the empirical formula of hydrated copper (II) sulphate is “CuSO4. 5H2O”, which is deemed correct by many people. But to be more precise, what CuSO4. 5H2O implies is that in the lattice of the CuSO4 molecule, there are on average 5 H2O molecules “trapped” in the lattice structure, which is not true, as experimentally determined. But through experiments the empirical formula of hydrated copper (II) sulphate is not CuSO4. 5H2O but rather [Cu (H2O)4]SO4. 5H2O. Note that in this formula, it clearly shows that the Cu2+ ion is bonded to 4 H2O molecules, and in the whole crystal one H2O molecule is trapped per every [Cu (H2O)4]2+ and SO42- ion. That is why although there is a compound called CrCl3. 6H2O, there are actually 3 different salts of it, known as isomers, with different colours in solution and even chemical properties.
For a compound/ion to be a ligand it must most obviously have a lone pair (or else how would it ever form a coordinate covalent bond?). They are Lewis bases (remember? Lewis bases are electron-pair donors), and the most common ligand is H2O and NH3. There are many more of them, like CN- and S2O32-, and I will be elaborating later about some properties of them (in the 2nd part).
Now back to the transition metal ion. I believe you know how an s-orbital looks like? A p-orbital? To refresh your minds:
(Pictures generously taken from http://en.wikipedia.org/wiki/Atomic_orbital (since it is free to take, as stated so in the license agreement))
Let’s focus on the d-orbitals. Notice the shapes of the orbitals. All of them actually look nearly the same except 2. (Pictures from www.chemsoc.org/VISELEMENTS/orbital/orbital_d.html) In order- from dxy to dxz to dyz to dx2-y2 to dz2:
The 1st thing that is obvious is that the 3dz2 orbital looks different from the other 4 orbitals-it has only 2 lobes, both on the z-axis, and an inner region of high electron density. Next, one can also realize that the 3dxy, 3dxz and 3dyz orbitals look exactly the same except for the axes (all have lobes of high electron density in between the axes), while the 3dx2-y2 orbital has its 4 lobes (regions of high electron density) all on the x and y axes. This will come in important later on.
Now lets try to figure out the significance in the shape of the orbitals with respect to the energy level. These are the orbitals binding to the electron pairs of the ligands, and as known, the lower the energy level the more stable the bond formed. Since in an isolated ion (aka no ligands) the d atomic orbitals are degenerate (have the same energy levels), thus one will expect it to be degenerate also when bonded to ligands. Not true. When the electron pair in the ligands bond, for the dx2-y2 and the dz2 orbitals the electron is forced to go near the region of higher electron density (since the electron pair of the ligands bond at the axes), thus it will experience more electrostatic repulsion, and thus the energy of the electron increases due to like charges.
Labels: chem
{ 9:55 PM }
Ok this week was mad.
MathRA CCT and phy CCT went well I suppose. I screwed up a 2mark qn but I suppose I can get 28 for math, while for phy I got the 5 mark bonus, but lost 2 marks, so I hope I still can get full marks. Geog and SS CCT marks were decent, but could have done better, 85 and 87 respectively.
Today was pure mad. During fire drill we sat under the sun for 40 min, and I had my phy notes with me. I didn’t really feel like mugging anymore, so I was looking around, and saw Keng Yong beside me gathering sand, and I decided to join him. Keng Yong, Mark and I ended up building a marvelous sandcastle at the end of the drill.
Anyway in the afternoon the RISE EXCO ’08 went to
Anyway by
Labels: school
{ 8:35 PM }
Tuesday, August 28, 2007.
I never knew the whole Chem RA class wants to kill me.Aim the NO2 at me lar! What is this? (joking lar)
Anyway Mr Ong decided that in order to go up to Sec 4 ChemRA we needed to post sth on our RA blog. So by Thurs I shall happily send my article on CFT (crystal field theory) to Mr Ong, since it is real useful during DMP. I target it to be readable and interactive, aka worksheets and problems. Accidentally found one problem online-the International Chem Olympiad '06 practice paper has a qn on CFT, guess I'll put it in too. And I decided in addition I shall do something abt quad bonding and stuff, since it is so interesting.
THAT IS IF I SURVIVE TMR AND THURS.
Z^n=1
Z= cos (2k (pi) /n) + i sin (2k (pi) /n ), k = 0, 1, 2, ..., n-1.
ARRGH maths.
Labels: school
{ 9:49 PM }
Monday, August 27, 2007.
Dear all-I LURVE RISE EXCO '08!I couldn't have hoped for a better EXCO. Wang Kai Cheng (Chairman, responsible 3A monitor), Yeo Shang Xuan (Vice-Chairman, Prefect of 3D), Me (Quartermaster), Low Zhao Kai (Librarian, 3D's "Pamela Anderson"), Chu Ben Wee (Treasurer, 3A resident violinist). Wonderful guys, WONDERFUL.
Anyway we had lunch treated by the great and revered Mr Sze, and spent a lot of time discussing EXCO matters. We were so freaking full, I promptly skipped dinner. My precious Maths and Physics mugging time are gone (wasted 6 hrs there) but I am willing to sacrifice (aiyah my Math RA marks are dead, so might as well), and I loved lunch-Fish and Co.'s double platter is gigantic.
Anyway, to end it all up: "Welcome to RISE EXCO."-Mr Sze
Labels: CCA
{ 7:34 PM }
Sunday, August 26, 2007.
Yesterday i saw this particular CD while searching online for shostakovich violin concerto1.

Historic Russian Archives - David Oistrakh [Box set]
http://www.amazon.co.uk/Historic-Russian-Archives-David-Oistrakh/dp/B0009OALK6/ref=sr_1_18/203-8618017-1671153?ie=UTF8&s=music&qid=1188134949&sr=1-18
Yeah RIGHT. Dream on, Lumpy, it costs at least 90 Sing bucks.
BUT-BUT-BUT-
It has EVERYTHING (save a few, which cbw won't approve of). Both Shostakovich, Beethoven, Lalo, Tchaikovsky, Mendelssohn, you name it, it has.
Anyway my "Les introuvables de David Oistrakh" (4 CD set-25 Sing bucks at HMV Citylink) is a steal. So I'm not sure whether I should forgo buying dozens of chemistry textbooks and A-level practice papers for such a wonderful set.
Stop dreaming, Lumpy, and go back to mugging maths and physics. "Whacks head" "Pinch"
Labels: Music
{ 9:20 PM }
Saturday, August 25, 2007.
I feel shocked.So, Shawn won. Apparently I have no feelings about it, unlike the past. Why?
I was walking to get a drink, and I saw snippets of the post-CSS2 celebrations. I asked my mother about the results, and I felt nothing. Maybe I don't know anyone from there, but I suspect it's something else.
Have I lost all my love for any other kind of music? I Youtubed Rihanna's "Umbrella", and I couldn't stand it. I feel worried-I want to have a little love for most other kinds of music.
I admit I did not care about Marcus at all, and I felt only a tinge of sadness for him when he left. Perhaps if he went for any other competition I would have shown more support for him (hint-NSC), but I guess it's the presence of non-classical music which scares me.
难道我变成了一个受不了其它音乐的怪兽吗?我希望不是。
Labels: Music
{ 11:01 PM }
I FINALLY gave in to the power of blogging. I should REALLY hate myself for this, really.
I never wanted to blog for years, but then my mind went haywire and told me to blog, so this is it-the result of chemistry damage to one's brain.
Anyway, I will be looking forward to posting more and more as I enter the blogosphere-I just can't shut up (coughCHEMRAcough).
quote huiyao:
"i am random, hear me moo. i weigh twice as much as you","and i look good with a chem textbook","i can recite the periodic table or tell you VSEPR. i am random hear me moo"
that's it for today-
よろしくお願い致します。
Labels: blog
{ 9:27 PM }
narcissism.
lumpy.
4B '08, RISE
RJCE, Alchemy
materialist.
oh am I? *scratches head*
music.
shostakovich. mahler. brahms. rachmaninoff. vaughan williams. bruckner. bach. tchaikovsky.
はなせ.
でぐち.
4B '08!
RISE!
Others
memories.
August 2007 September 2007 October 2007 November 2007 December 2007 January 2008 February 2008 March 2008 April 2008 May 2008 June 2008 July 2008 August 2008 September 2008 October 2008 November 2008 December 2008 January 2009 February 2009 March 2009 April 2009 May 2009 June 2009 July 2009 August 2009 September 2009 October 2009 November 2009 December 2009 January 2010 March 2010
thanks.
Layout by BAKEDPOTATOE, with help from sm3no for the image and fonts, Print Dashed and Violation.